The first-ever year-long* systemic flea and tick protection for dogs

A revolutionary injectable with a trusted ingredient, fluralaner

Available exclusively through veterinary practitioners



A VETERINARY-EXCLUSIVE
Designed to be the ultimate support to you and your efforts to control parasite infestations and vector-borne diseases.
CERTAINTY
Delivering the injection in the clinic ensures fewer gaps in protection, eliminating reliance on pet owners to administer products correctly, at the proper frequency.
CONVENIENCE
BRAVECTO injectable is administered as a single injection during a veterinary visit once a year, at any time of year.
COMFORT
The innovative suspension of fluralaner crystals allows for smooth administration and therefore a favourable patient and owner experience.


KEEP PATIENTS PROTECTED FOR A FULL YEAR*
BRAVECTO injectable
protects dogs 6 months of age or older against multiple species of fleas and tick, including:

Ctenocephalides felis
Ctenocephalides canis

Ixodes ricinus
Ixodes hexagonus
Dermacentor reticulatus
Rhipicephalus sanguineus
BRAVECTO injectable delivers year-long protection*
Pharmacokinetics
PHARMACOKINETICS
Plasma fluralaner concentrations following a single subcutaneous administration1
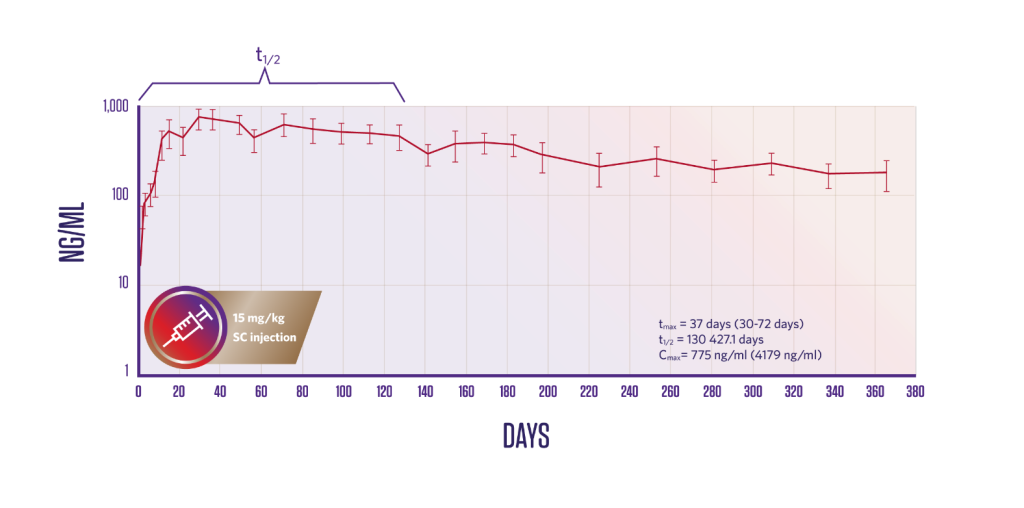
*Local indications may vary. Please see product leaflet for complete details.
Flea efficacy

IMMEDIATE AND PERSISTENT FLEA-KILLING ACTIVITY FOR 12 MONTHS
Duration of efficacy against Ctenophalides felis – adulticide2*
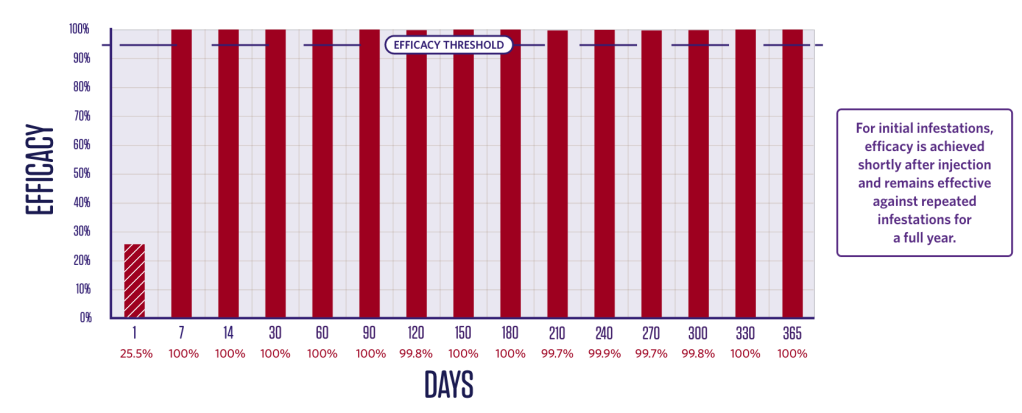
Newly infested fleas killed within 24 hours.2,3

2 groups (1 treated, 1 control);
10 dogs in each group.

Dogs were infested with 100 fleas on Day -1 and treated on Day 0. Efficacy against the initial infestation was evaluated on Day 1.

Persistent efficacy evaluated at
intervals from Day 7 to Day 366
following repeated re-infestations.
*Flea counts are least squares means. Percent effectiveness is based on least squares means.

NEWLY EMERGED FLEAS ARE KILLED BEFORE VIABLE EGGS ARE PRODUCED
Efficacy against C felis – egg production2*
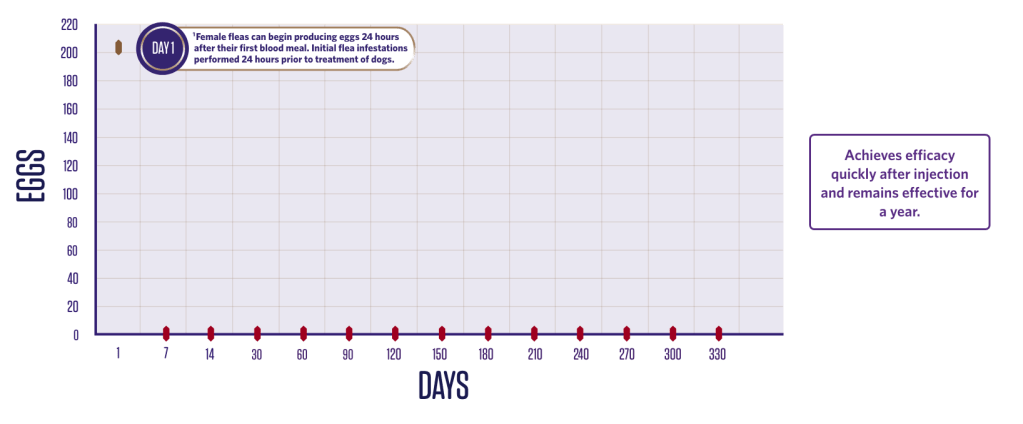
The flea lifecycle is broken due to the rapid onset of action and long-lasting efficacy against adult fleas on the animal and the absence of viable egg production.3

2 groups (1 treated, 1 control);
10 dogs in each group.

Dogs were infested with 100 fleas on Day -1 and treated on Day 0. Efficacy against the initial infestation was evaluated on Day 1.

Persistent efficacy evaluated at
intervals from Day 7 to Day 366
following repeated re-infestations.
*Flea egg counts are arithmetic count
*Local indications may vary. Please see product leaflet for complete details.
Tick efficacy

PERSISTENT TICK KILLING ACTIVITY FOR 12 MONTHS
Duration of efficacy against Rhipicephalus sanguineus4*
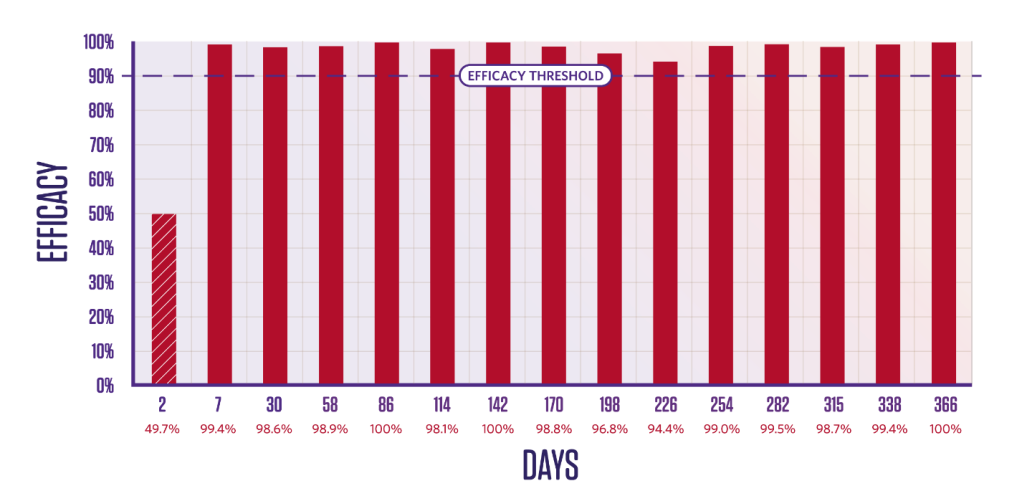
Persistent Rhipicephalus sanguineus tick-killing activity from Day 4 after treatment for 12 months. Newly infested R. sanguineus ticks are killed within 48 hours.3,4

2 groups (1 treated, 1 control);
10 dogs in each group.

Dogs were infested with 50 ticks on Day -2 and treated on Day 0. Efficacy against the initial infestation was evaluated on Day 2.

Persistent efficacy evaluated at intervals from Day 7 to Day 366 following repeated re-infestations.
*Tick counts are arithmetic count

PERSISTENT TICK KILLING ACTIVITY FOR 12 MONTHS
Duration of efficacy against Dermacentor reticulatus5
Persistent Dermacentor reticulatus killing activity from Day 3.
Newly infested D. reticulatus ticks are killed within 48 hours.3,5

2 groups (1 treated, 1 control);
10 dogs in each group.

Dogs were infested with 50 ticks on Day -2 and treated on Day 0. Efficacy against the initial infestation was evaluated on Day 2.

Persistent efficacy evaluated at intervals from Day 7 to Day 366 following repeated re-infestations.
*Tick counts are arithmetic count

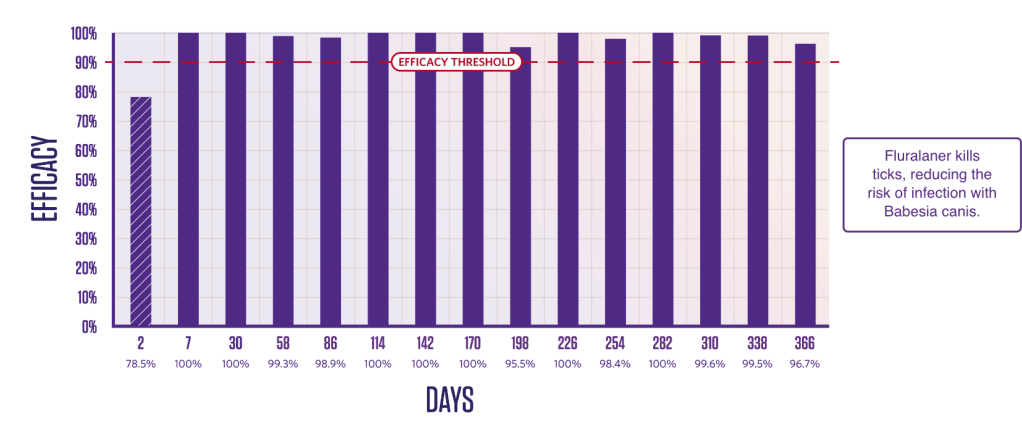
PERSISTENT TICK KILLING ACTIVITY FOR 12 MONTHS
Duration of efficacy against Ixodes ricinus6,7
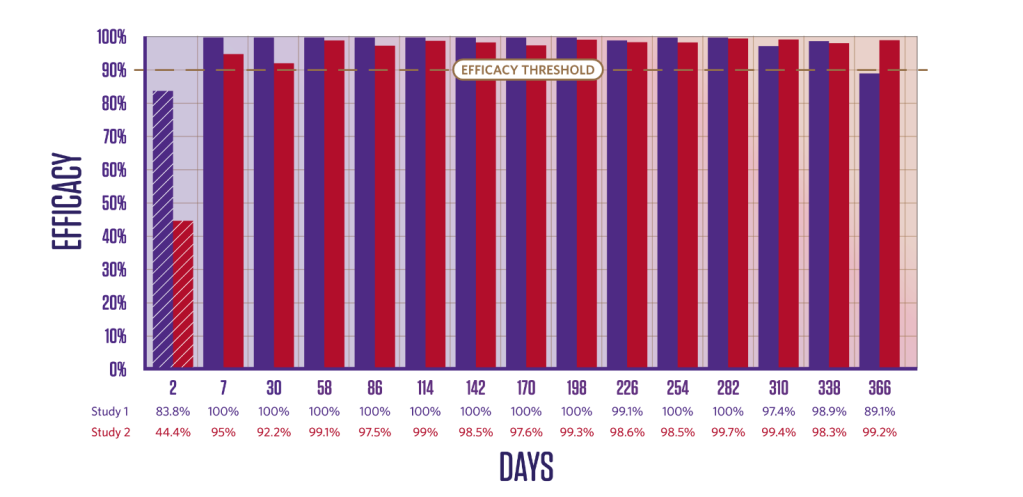
Persistent Ixodes ricinus killing activity from Day 3.
Newly infested I. ricinus ticks are killed within 48 hours.3,7

2 groups (1 treated, 1 control);
10 dogs in each group.
Dogs were infested with 50 ticks on Day -2 and treated on Day 0. Efficacy against the initial infestation was evaluated on Day 2.
Persistent efficacy evaluated at intervals from Day 7 to Day 366 following repeated re-infestations.
*Tick counts are arithmetic count
*Local indications may vary. Please see product leaflet for complete details.


PRECISE DOSING

Multi-dose vial allows you to draw the exact dose you need for dogs of any size (15 mg fluralaner/kg body weight; 1ml/10kg body weight).

3 year shelf life of
BRAVECTO injectable,
3 months at room temperature after reconstitution.

For dogs from 6 months of age.
Discover our BRAVECTO Chew for puppies from 8 weeks of age.


HOW TO PREPARE FOR USE?
To ensure proper product preparation, follow the step-by-step mixing instructions carefully.


What vets involved in clinical field studies say about Bravecto INJECTABLE
“It is a highly effective product, and to date, we had nothing on the market offering good antiparasitic (flea and tick) protection for a year.“
“When I told the pet owners about the medication, they were excited and the question that was often asked was when it would be available.“


ADDRESSING PET OWNER’S QUESTIONS ABOUT BRAVECTO INJECTABLE
Pet owners may have questions. Download our guide that helps answer commonly asked questions by pet owners who are considering using BRAVECTO injectable.
UNLEASH THE POWER OF BRAVECTO INJECTABLE

Experience a unique formulation of fluralaner, one of the world’s most trusted flea and tick medications.
- 12 months* of non-stop protection.3
- Revolutionary injectable formulation of a trusted ingredient.
- Veterinary exclusive.
It is designed for veterinary administration to deliver compliance and convenience.
FREQUENTLY ASKED QUESTIONS
Due to the possibility of fast-growing puppies outgrowing the dose, the product was developed for dogs and puppies 6-months of age and older.
There is no contra-indication to use concurrently with vaccination. Vets should keep in mind vaccinations may cause fever, lethargy and inappetence, occurring within the first few days following vaccination.
Because the efficacy is sustained for an entire year following administration, dogs can be treated whenever they are unprotected against fleas and ticks. Dogs will not require another dose of flea and tick treatment for 12 months* for the indicated parasites.
A margin of safety study was performed and demonstrated safety at doses of 1X (15 mg/kg), 3X (45 mg/kg) and 5X (75 mg/kg) the standard therapeutic dose administered every 4 months for a total of 6 doses. The only treatment-related finding was limited to injection site swellings that resolved over time.
To maintain a uniform suspension and accurate dosing, the dose should be administered within approximately 5 minutes after drawing it into the dosing syringe.
The formulation is a suspension containing fluralaner powder and an 18 G needle is required to ensure the solid particles in suspension are accurately transferred from the vial into the syringe and from the syringe into the patient.
Dogs should be dosed at a rate of 0.1 ml per kg of bodyweight. Dogs weighing 10 kg should be injected with 1.0 ml; dogs weighing 15 kg should be injected with 1.5 ml, etc.
To maintain efficacy of BRAVECTO injectable dogs should be re-dosed 12 months* after the previous injection.
The sterilisation process used for the powder causes the glass to darken in colour, which is not the case for process used to sterilise the liquid vehicle.
After reconstitution, the suspension must be discarded within 3 months from the date of reconstitution. Write the discard date on the label of the glass vial.
Request further information to bring innovative flea and tick protection to your practice
We will answer your questions to ensure you are ready to prescribe Bravecto Injectable, putting the power of flea and tick protection back in your hands.
“*” indicates required fields


Discover our whole Bravecto franchise
REFERENCES
- Data on file, Study Summary REF-10145, MSD Animal Health.
- Data on file, Study Summary REF-10157, MSD Animal Health.
- BRAVECTO 150mg/ml powder and solvent for suspension for injection for dogs. Summary of Product Characteristics. Product information published on EMA webpage.
- Data on file, Study Summary REF-10166, MSD Animal Health.
- Data on file, Study Summary REF-10142, MSD Animal Health.
- Data on file, Study Summary REF-10143, MSD Animal Health.
- Data on file, Study Summary REF-10161, MSD Animal Health.
*Local indications may vary. Please see product leaflet for complete details.



